“Did you know? October - December 2025"
Did you know ……….that polymer molecular weights (MW) produced in emulsion polymerization can be extremely high when no chain transfer agent is used? Did you also know that the type of process used (batch, semi-batch) can significantly affect the polymer MW? This issue of our “Did you know….” presentations is the second in a multi-part series that will collectively discuss the molecular weights typically produced in both batch and semi-batch emulsion polymerization (EP) processes, the effectiveness of chain transfer agents (CTA) in both process types, and the possibility of polymer chain branching and cross-linking in the latex particles. In this issue we address the molecular weight development in batch EP when some level of CTA is used.
In general, linear polymer chain lengths produced in free radical polymerizations depend upon the rate of propagation of the polymer radical and the rate at which chain growth is stopped (termination, transfer), the so-called initiation, propagation, termination sequence. This is certainly true in EP. Here we have latex particles constantly receiving oligomeric radicals (typically containing ~3-6 monomer units) from the aqueous phase – some grow into high MW polymer and some terminate other polymer radicals already in the particles. During the first two time intervals in batch EP (particle nucleation and particle growth), the monomer concentration in the particles (the driver of chain length growth) is essentially constant and the polymer radical chains grow at a constant rate. The stopping events happen when a second free radical enters a particle that contains a growing polymer chain and terminates the growing chain or when the CTA reacts with a growing radical chain in the particle. The CTA simply adds to the frequency of chain stopping events and thus produces lower MWs. This is also true in the third time interval when the monomer concentration in the particles decreases continually until final conversion.
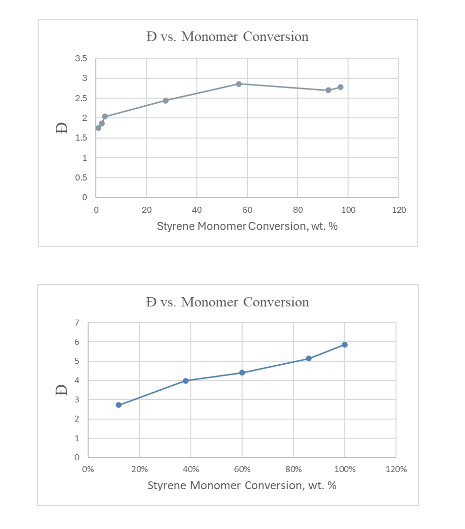
The CTA has its biggest effect during the highest MW producing time intervals of the reaction (especially interval 2) so as to bring those chain lengths closer to the shorter ones produced during the third time interval. This leads to an overall lowering of the MWs produced over the course of the batch reaction. In addition, and importantly, the breadth of the distribution (dispersity, Ð = Mw/Mn) is substantially lowered. Thus it is no wonder that CTAs have been so commonly used in emulsion polymerizations.
The first plot below shows how the dispersity proceeds with time (expressed as conversion of monomer to polymer) for a typical polystyrene batch emulsion polymerization at 70 °C when a common level of CTA (t-dodecyl mercaptan in this case) is used. You will note that compared to the results when no CTA is used (the second, or lower plot), there is a remarkable narrowing of the MW distribution. This, as explained above, is due to the lowering of the MW produced during the first two-time intervals of the EP reaction, lessening the impact of the lower MW chains produced during the third time period.
We invite your questions and comments via our website, epced.com


